












This is a single-use type where the energy will gradually run out as you use them. This is the most popular type and the one most people first think of when we talk about batteries.
So, how is electricity produced inside the battery? Let’s look at a simple experiment.
This is a single-use type where the energy will gradually run out as you use them. This is the most popular type and the one most people first think of when we talk about batteries.
So, how is electricity produced inside the battery? Let’s look at a simple experiment.

1. Electrons generated on zinc plate

Electrons are generated on the zinc plate. The zinc atoms which make up the zinc plate leave out some spare electrons, creating zinc irons which break down in the electrolyte solution. The copper plate hardly breaks down at all.
2. Mass transfer of electrons from zinc plate

The number of electrons on the zinc plate increases the more the zinc breaks down. These electrons then transfer to the positive copper plate via conduction.
3. Hydrogen ions accept electrons

The sulfuric acid which makes up the electrolyte solution contains hydrogen ions. However, when zinc ions appear in the same electrolyte solution, hydrogen is weaker at forming ions than zinc, so the hydrogen ions bond with the electrons travelling to the copper plate and return to a hydrogen gas state.
Manganese battery
![]()
Nominal voltage
1.5 V
In this structure, the negative (-) pole also serves as the container. This means that holes can open up in the container and fluid spill out due to excessive discharge if accidentally left on for too long. So we need to be careful. These batteries have a long history and are cheap and popular. When left off, they can recover some of their energy, so they are ideal for things we use occasionally like flashlights or things we use repeatedly but only for short periods each time.

Alkaline battery
![]()
Nominal voltage
1.5 V
In this structure, the outer container has nothing to do with the chemical reaction so there is little risk of leakage. These alkaline batteries have higher capacity and less voltage reduction than manganese batteries, so they are suited for things that need powerful currents like bright lights, and things we use for long periods at a time like portable stereos.

Primary lithium battery
![]()
Nominal voltage
3.0 V
This type of battery offers high performance, featuring high voltage and reliability, and a maximum amount of energy per volume that can be as high as ten times that of manganese dry batteries. Its electrolyte contains no water, allowing for use at low temperature. This battery comes in various forms and is of great use in a variety of applications, for example, a coin type for digital clocks, a pack type for cameras, and a pin type for fishing floats.
Alkaline button battery
![]()
Nominal voltage
1.5 V
This is a high-performance and low-price battery used in electronic toys, calculators, cameras, etc.
Silver oxide battery
![]()
Nominal voltage
1.55 V
This type has a large capacity, and its voltage is stable. Its long life is worth the high price. Many button type batteries, for example those used for watches, are of this type. Some of these batteries are 2 mm or less in thickness and ideal for precision equipment.
Zinc-air battery
![]()
Nominal voltage
1.4 V
These are used in things like hearing aids in place of mercury batteries. They cannot be used in sealed devices where air cannot get inside. The generation of electricity starts when the seal is removed.
This is a single-use type where the energy will gradually run out as you use them. This is the most popular type and the one most people first think of when we talk about batteries.
So, how is electricity produced inside the battery? Let’s look at a simple experiment.
This is a single-use type where the energy will gradually run out as you use them. This is the most popular type and the one most people first think of when we talk about batteries.
So, how is electricity produced inside the battery? Let’s look at a simple experiment.

1. Electrons generated on zinc plate

Electrons are generated on the zinc plate. The zinc atoms which make up the zinc plate leave out some spare electrons, creating zinc irons which break down in the electrolyte solution. The copper plate hardly breaks down at all.
2. Mass transfer of electrons from zinc plate

The number of electrons on the zinc plate increases the more the zinc breaks down. These electrons then transfer to the positive copper plate via conduction.
3. Hydrogen ions accept electrons

The sulfuric acid which makes up the electrolyte solution contains hydrogen ions. However, when zinc ions appear in the same electrolyte solution, hydrogen is weaker at forming ions than zinc, so the hydrogen ions bond with the electrons travelling to the copper plate and return to a hydrogen gas state.
Manganese battery

Nominal voltage
1.5 V
In this structure, the negative (-) pole also serves as the container. This means that holes can open up in the container and fluid spill out due to excessive discharge if accidentally left on for too long. So we need to be careful. These batteries have a long history and are cheap and popular. When left off, they can recover some of their energy, so they are ideal for things we use occasionally like flashlights or things we use repeatedly but only for short periods each time.

Alkaline battery

Nominal voltage
1.5 V
In this structure, the outer container has nothing to do with the chemical reaction so there is little risk of leakage. These alkaline batteries have higher capacity and less voltage reduction than manganese batteries, so they are suited for things that need powerful currents like bright lights, and things we use for long periods at a time like portable stereos.

Primary lithium battery

Nominal voltage
3.0 V
This type of battery offers high performance, featuring high voltage and reliability, and a maximum amount of energy per volume that can be as high as ten times that of manganese dry batteries. Its electrolyte contains no water, allowing for use at low temperature. This battery comes in various forms and is of great use in a variety of applications, for example, a coin type for digital clocks, a pack type for cameras, and a pin type for fishing floats.

Alkaline button battery

Nominal voltage
1.5 V
This is a high-performance and low-price battery used in electronic toys, calculators, cameras, etc.
Silver oxide battery

Nominal voltage
1.55 V
This type has a large capacity, and its voltage is stable. Its long life is worth the high price. Many button type batteries, for example those used for watches, are of this type. Some of these batteries are 2 mm or less in thickness and ideal for precision equipment.
Zinc-air battery

Nominal voltage
1.4 V
These are used in things like hearing aids in place of mercury batteries. They cannot be used in sealed devices where air cannot get inside. The generation of electricity starts when the seal is removed.
These batteries are very convenient as they can be recharged and used again after their energy has originally run out. They are used in many small devices such as mobile phones and are now being deployed in an ever wide range of fields.


Ni-Cd battery
![]()
Nominal voltage
1.2 V
In this structure, the gas generated through the chemical reaction during charging can be absorbed internally. All rechargeable batteries are built this way. However, when not in use they will naturally discharge and the power will run out in 3-6 months, so we should charge them fully before use.

Nickel-metal hydride battery
![]()
Nominal voltage
1.2 V
Like Ni-Cd batteries, these can be recharged and used again. The structure is similar to Ni-Cd batteries too, but these have a higher capacity and can be used continuously for 50-100% longer. This makes them ideal for devices we used over long periods, such as digital cameras.
Lithium-ion battery
![]()
Nominal voltage
3.7 V
This is a new type of batteries which arrived in the 1990s and replaced metallic lithium with lithium ions. Lithium-ion batteries are lighter than Ni-Cd or nickel-metal hydride batteries and can be used for longer periods. Their self-discharge rate is also lower, and they do not suffer from memory effect.
Lead acid storage batteries
These secondary batteries have the longest history of all. They are used in cars and motorbikes, while the “sealed” value-regulated variety is used as a backup or emergency power source for things like hospitals, factories, buildings and computers.


These batteries are very convenient as they can be recharged and used again after their energy has originally run out. They are used in many small devices such as mobile phones and are now being deployed in an ever wide range of fields.
These batteries are very convenient as they can be recharged and used again after their energy has originally run out. They are used in many small devices such as mobile phones and are now being deployed in an ever wide range of fields.


Ni-Cd battery

Nominal voltage
1.2 V
In this structure, the gas generated through the chemical reaction during charging can be absorbed internally. All rechargeable batteries are built this way. However, when not in use they will naturally discharge and the power will run out in 3-6 months, so we should charge them fully before use.

Nickel-metal hydride battery

Nominal voltage
1.2 V
Like Ni-Cd batteries, these can be recharged and used again. The structure is similar to Ni-Cd batteries too, but these have a higher capacity and can be used continuously for 50-100% longer. This makes them ideal for devices we used over long periods, such as digital cameras.
Lithium-ion battery

Nominal voltage
3.7 V
This is a new type of batteries which arrived in the 1990s and replaced metallic lithium with lithium ions. Lithium-ion batteries are lighter than Ni-Cd or nickel-metal hydride batteries and can be used for longer periods. Their self-discharge rate is also lower, and they do not suffer from memory effect.
Lead acid storage batteries
These secondary batteries have the longest history of all. They are used in cars and motorbikes, while the “sealed” value-regulated variety is used as a backup or emergency power source for things like hospitals, factories, buildings and computers.

How do we generate electricity from sunlight? Let’s look at the example of this “solar cell” (or “photo cell”). Solar cells are batteries which turn light energy from the sun into electrical energy. Whether in the mountains or at sea, they can easily be used to generate electricity wherever there is sunlight without causing any pollution or disturbance. The principle behind solar cells involves joining together a P-type semiconductor with negative electrical properties. When the sunlight hits a contact point on the P-type semiconductor, both positive and negative properties are collected at both ends of the battery, generating voltage and electrical energy.
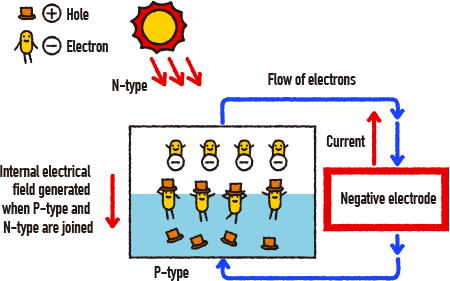

How do we generate electricity from sunlight? Let’s look at the example of this “solar cell” (or “photo cell”). Solar cells are batteries which turn light energy from the sun into electrical energy. Whether in the mountains or at sea, they can easily be used to generate electricity wherever there is sunlight without causing any pollution or disturbance. The principle behind solar cells involves joining together a P-type semiconductor with negative electrical properties. When the sunlight hits a contact point on the P-type semiconductor, both positive and negative properties are collected at both ends of the battery, generating voltage and electrical energy.

What is a fuel cell? Let’s take a look at this simple diagram. The molecular formula for water is H2O. This means that it is made from hydrogen and oxygen. When electrical current passes through water, this generates both hydrogen and oxygen gas. This is called “electrolysis” of water. Fuel cells use this electrolysis process in reverse – in other words, when hydrogen and oxygen are bonded, this generates water and electricity.
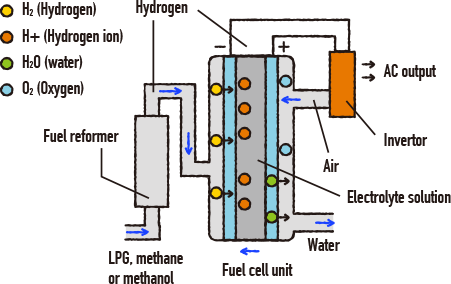

What is a fuel cell? Let’s take a look at this simple diagram. The molecular formula for water is H2O. This means that it is made from hydrogen and oxygen. When electrical current passes through water, this generates both hydrogen and oxygen gas. This is called “electrolysis” of water. Fuel cells use this electrolysis process in reverse – in other words, when hydrogen and oxygen are bonded, this generates water and electricity.


















